-


CO2 Laser Tube CE Certification
Submission Dote: August 5, 2019Reci CO2 Laser Tube is in compliance with the council LVD Directive 2014/35/EU and obtained the CE Certification on August 5, 2019. It is possible to use CE marking to demonstrate the compliance with this LVD Directive.
-


CO2 Laser Tube RoHS Certification
Submission Dote: April 17, 2019Reci CO2 Laser Tube fulfil test requirement of the RoHS Directive(EU) 2015/863 and obtained the RoHS Certification on April 17, 2019.
-


CO2 Laser Tube FDA Certification
Submission Dote: September 8, 2021Reci CO2 Laser Tube is registered with the U.S. Food and Drug Administration pursuant to Title 21, Code of Federal Regulations(CFR), Part 1002 on September 8, 2021.
-


RF Laser CE Certification
Submission Dote: November 24, 2020Reci RF Laser is in compliance with the council MD Directive 2006/42/EC and EMC Directive 2014/30/EU and obtained the CE Certification on November 24, 2020.
CCT Testing acknowledges Reci may issue a COC apply the CE marking in accordance with European Union Rules.
-


Air-cooled Fiber Laser FDA Certification
Submission Dote: December 28, 2021Reci Air-cooled Fiber Laser is registered with the U.S. Food and Drug Administration pursuant to Title 21, Code of Federal Regulations(CFR), Part 1002 on December 28, 2021.
-
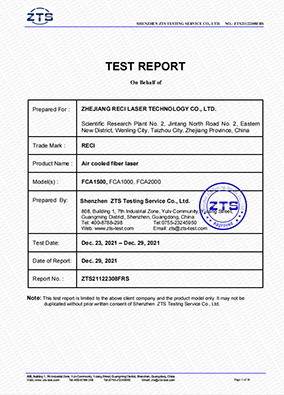

Air Cooled Fiber Laser IEC Certification
Submission Dote: December 29, 2021Reci Air Cooled Fiber Laser has been tested and found in compliance with IEC 60825-1:2014 and obtained the IEC Certification issued by Shenzhen ZTS Testing Service Co., Ltd. On December 29, 2021.
-


Air-Cooled Fiber Laser RoHS Certification
Submission Dote: December 28, 2021Reci Air-Cooled Fiber Laser has been tested and found in compliance with RoHS Directive 2011/65/EU and its subsequent amendments Directive (EU)2015/863. Reci obtained the Air-Cooled Fiber Laser RoHS Certification issued by Shenzhen ZTS Testing Service Co., Ltd. On December 28, 2021.
-


Air Cooled Fiber Laser CE Certification
Submission Dote: December 29, 2021Reci Air Cooled Fiber Laser is in compliance with the council LVD Directive 2014/35/EU and obtained the CE Certification on December 29, 2021. ZTS Testing acknowledges that Reci may issue a COC apply the CE marking in accordance with European Union Rules.
-


RF Laser
Submission Dote: January 1,2022Lorem ipsum dolor sit amet, consectetur adipiscing elit, sed do eiusmod tempor incididunt ut labore et dolore magna aliqua. Quis ipsum suspendisse ultrices gravida. Risus commodo viverra maecenas accumsan lacus vel facilisis. Lorem ipsum dolor sit amet, consectetur adipiscing elit, sed do eiusmod tempor incididunt ut labore et dolore magna aliqua. Quis ipsum suspendisse ultrices gravida. Risus commodo viverracc lacus vel facilisis. Lorem ipsum dolor sit amet, consectetur adipiscing elit, sed do eiusmod tempor incididunt uQuitriceen.
-


Fiber Laser Source CE Certification
Submission Dote: July 28, 2020On July 28, 2020, Beijing Reci Laser Technology Co., Ltd obtained the CE Certification of fiber lasers ource.
-


Fiber Laser FDA Certification
Submission Dote: September 8, 2021Reci Fiber Laser is registered with the U.S. Food and Drug Administration pursuant to Title 21, Code of Federal Regulations(CFR), Part 1002 on September 8, 2021.



































